4.4.2 Chemical Properties of Group 1 Elements
Chemical Properties of Alkali Metals
- Group 1 metals are very reactive metals.
- They all show the same chemical properties.
- They can react with water and non-metal such as oxygen and chlorine to form a new compound.
- The table to the right shows the electron arrangement of all the Group 1 metals. All the atoms of Group 1 metal consist of 1 valence electron.
- When an alkali metal atoms react, it loses the valence electron to form a positively charged ion.
Example:
- Li → Li⁺ + e
- Na → Na⁺ + e
- K → K⁺ + e
They tend to react mainly with non-metals to form ionic compounds.
Safety Precaution
- Alkali metals are very reactive.
- Therefore it must be kept in paraffin oil to prevent them from reacting with oxygen and water vapour in the air.
- We must avoid holding group 1 metals with bare hand because they may react with water on our hand.
- We must wear safety goggles and gloves during handling experiment involving group 1 metal.
Reaction of Alkali Metals with Chlorine
- All alkali metals react with chlorine gas to form white metal chlorides salt.
Group 1 Metals + Chlorine Gas → Metal Chloride - The metal chlorides salt formed is soluble in water to give a neutral solution of pH 7.
- The reactivity increases down the group from lithium, sodium to potassium.
Example:
Lithium + Chlorine
Observation
Lithium burned slowly with a reddish flame . A white solid is produced.
Sodium + Chlorine
Observation
Sodium burned brightly with a yellowish flame. A white solid is produced.
Potassium + Chlorine
Observation
Potassium burned very brightly with a purplish flame. A white solid is produced.
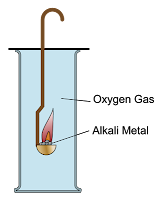
Reaction of Alkali Metals with Oxygen
- Group 1 metals react with oxygen gas produces metal oxides. These metal oxides dissolve in water produces alkalis.
Group 1 Metals + Oxygen Gas → Metal Oxide - Lithium, sodium and potassium form white oxide powders after reacting with oxygen.
- The white powder is the oxide of lithium, sodium and potassium.
- When the white powder is dissolved in water, it produces a solution which turned red litmus paper blue. Which means, these oxides dissolve in water to form strong alkali.
- The reactivity increases down the group from lithium, sodium to potassium.
Example
Lithium + Oxygen
Dissolve in water
Observation
Lithium burns with red flame and produces white powder immediately after reaction.
Sodium + Oxygen
Dissolve in water
Observation
Sodium burned with bright yellow flame, forming white powder immediately after reaction.
Potassium + Oxygen
Dissolve in water
Observation
Potassium burned with very bright purplish flame, forming white powder immediately after the reaction.
Reaction of Alkali Metals with Water
Group 1 metals react vigorously with water produces alkali and hydrogen gas
Common Observation
- Lithium, sodium or potassium floats and move around on the surface of the water and then dissolve in the water.
Conclusion:
Lithium, sodium and potassium are less dense than water. - Colourless gas is released around the metal. The gas produces a “pop” sound when ignited with a lighted wooden splinter.
Conclusion:
The colourless flammable gas is hydrogen. - The solution turns blue when it is tested with universal indicator.
Conclusion:
the solution produced is an alkali.
Lithium + Water
Observation
Lithium metal moves slowly on the surface of the water with ‘fizzing’ sound.
Sodium + Water
Observation
The lump of sodium moves swiftly on the surface of water with ‘fizzing’ sound
Potassium + Water
Observation
Potassium reacts violently with water, move very fast on the surface of water and burn with lilac flame.
Explaining the Reactivity Trend of the Alkali Metals
- When an alkali metal atom reacts, it loses its valence electron to form a positively charged ion.
Example
Li → Li+ + e
Na → Na+ + e
K → K+ + e - As we go down the group from one element down to the next, the atomic radius gets bigger due to an extra filled electron shell.
- The valence electron is further and further from the nucleus. Thus the attraction force between the nucleus and the valence electron become weaker and weaker.
- This causes the valence electron is easier to be released to form an ion when the atom takes part in a reaction.