01 Introduction to Chemistry
02 The Structure of Atoms
2.1 The Basic Concept of Matter
2 Lessons
03 Concept of Mole, Formulae and Equations
3.2 Concept of Mole
3 Lessons
3.3 Chemical Formulae
2 Lessons
04 Periodic Table of Elements
4.5 Group 17 Elements
2 Lessons
5.3 Covalent Bonds
1 Lesson
2.2.1 The History of Development of the Atomic Model
History of Development of the Model of Atom
Table below shows the scientists that contribute to the development of the Model of Atom.
John Dalton
|
|
J.J. Thomson 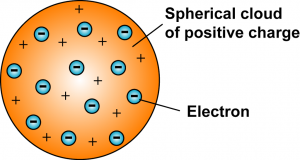 |
The electrons were positioned uniformly throughout the atom. |
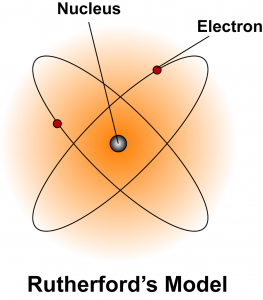
Ernest Rutherford
 |
|
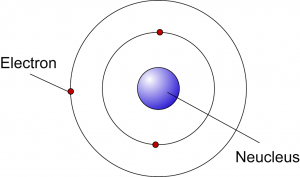
Neils Bohr
 |
|
James Chadwick
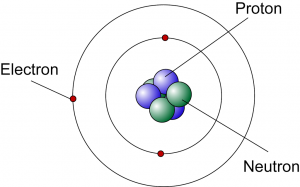 |
|