01 Introduction to Chemistry
02 The Structure of Atoms
2.1 The Basic Concept of Matter
2 Lessons
03 Concept of Mole, Formulae and Equations
3.2 Concept of Mole
3 Lessons
3.3 Chemical Formulae
2 Lessons
04 Periodic Table of Elements
4.5 Group 17 Elements
2 Lessons
5.3 Covalent Bonds
1 Lesson
4.4.1 Group 1 Elements
The Alkali Metals
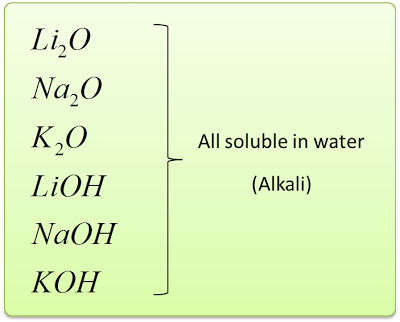
- The Group 1 metals is called the Alkali Metals.
- This is because they form oxides and hydroxides that dissolve in water to give alkaline solutions.
- As shown in the diagram on the right, elements in this group are lithium, sodium, potassium, rubidium, caesium and francium.
- They are the first element of a period, with one valence electron.
- This similarity (1 valence electron) makes them chemically behave in a similar manner.
- All alkali metals are very reactive. They must be stored in oil prevent reaction with oxygen or water vapour in air.
Physical Properties of Alkali Metals
| Name | Proton number | Electron arrangement |
| Lithium | 3 | 2.1 |
| Sodium | 11 | 2.8.1 |
| Potassium | 19 | 2.8.8.1 |
| Rubidium | 37 | 2.8.18.8.1 |
| Caesium | 55 | 2.8.18.18.8.1 |
| Francium | 87 | 2.8.18.32.18.8.1 |
- All Group 1 metal exist as solid at room temperature and hence have all the typical metallic properties, such as:
- good conductors of heat
- good conductors of electricity,
- high boiling points,
- shiny surface (but rapidly tarnished by air oxidation).
- Nevertheless, Group 1 metals also show some non-typical metallic properties, such as:
- low melting points,
- low density (first three floats on water),
- very soft (easily squashed, extremely malleable, can be cut by a knife).
Important trends down the group:
- size of atoms increases
- the melting point and boiling point decrease
- the density increases.
- the hardness decreases.
Size of Atom
- Down the group, the size of atom increases.
- This is due to the increase in the number of electron shells.
- An atom with more shells is bigger than an atom with fewer shells.
Boiling Point and Melting Point
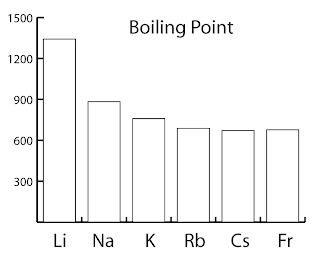
| Name | melting point | boiling point | Density g/cm3 |
| Lithium | 180ºC | 1342ºC | 0.53 |
| Sodium | 98ºC | 883ºC | 0.97 |
| Potassium | 63ºC | 759ºC | 0.86 |
| Rubidium | 39ºC | 688ºC | 1.48 |
| Caesium | 29ºC | 671ºC | 1.87 |
| Francium | 27ºC | 677ºC | > 1.87 |
- The melting point and boiling point generally decrease down the group.
- All the atoms of Group 1 metals are bonded together by a force called the metallic bond.
- The strength of the metallic bond depends on the distance between the atoms. The closer the atoms, the stronger the bond.
- Down the group, the size of the atoms increases, causing the distance of the atoms increases.
- As the distance between the atoms increases, the metallic bond between the atoms decreases.
- Therefore, less energy is needed to overcome the metallic bond during the melting process.
- Consequently, the melting point of Group 1 metal decreases down the group.
Density
- The densities of Group 1 metals are low compared with the other metals.
- The densities of the first 3 elements (Lithium, Sodium and Potassium) are lower than water. Thus, they can float on the surface of water.
- Nevertheless, the density increases steadily down the group.
- The density of a substance is given by the equation “Density=Mass/Volume”.
- Down the group, both the mass and the volume increase, but the increase of mass is faster than the volume, hence the density increases down the group