01 Introduction to Chemistry
02 The Structure of Atoms
2.1 The Basic Concept of Matter
2 Lessons
03 Concept of Mole, Formulae and Equations
3.2 Concept of Mole
3 Lessons
3.3 Chemical Formulae
2 Lessons
04 Periodic Table of Elements
4.5 Group 17 Elements
2 Lessons
5.3 Covalent Bonds
1 Lesson
2.1.1 The Particle Theory of Matter
The Particle Theory of Matter
- Matter is anything that occupies space and has mass.
- The particle theory of matter states that matter is made up of a large number of tiny and discrete particles.
Particle Theory of Matter:
Matter is made up of a large number of tiny and discrete particles.
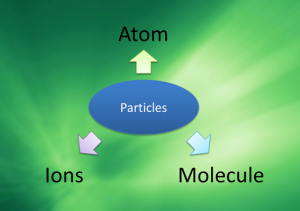
Types of Particles
- Particles can exist as atoms, molecules or ions.
- Atom is the smallest, indivisible particle of an element.
- Molecules are particles that made up of two or more atoms.
- Ions are particles that carry charge.
- Positive ion – Cation
- Negative ion – Anion

Diffusion and Brownian Motion
Proof of Particle Theory of Matter – Diffusion
In SPM, you need to know
- diffusion is one of the proofs of the particle theory of matter.
- the definition of diffusion.
- diffusion in solid, liquid and gas
- factors that affect the rate of diffusion and the related experiments.
What is Diffusion?
- Diffusion is a process of spreading a substance from a region of high concentration to a region of low concentration.
- It occurs when the particles of the substance move through the space between the particles of another substance.
- Figure below shows how the bromine particles diffuse into the air.

- Diffusion occurs in solid, liquid and gas.
- The rate of diffusion is highest in gas and lowest in solid.
- Diffusion is proof of the particle theory of matter.
MUST KNOW!
- The rate of diffusion is highest in gas and lowest in solid.
- Diffusion is the proof of the particle theory of matter.
Interesting Video
Diffusion in Solid
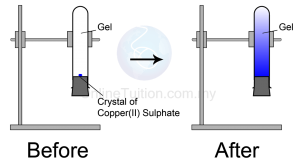 |
| Diffusion in Solid |
Observation
The blue colour of copper(II) sulphate fills up the entire test tube after a few days
- Copper(II) sulphate crystals are made of copper(II) ions and sulphate ions which are tiny and discrete.
- The particles in the copper(II) sulphate crystal will separate to become ions and diffuse randomly upwards until the whole agar turns blue.
Diffusion in Liquid
 |
| (Diffusion in Liquid) |
Observation
The purple colour of potassium manganate(VII) fills up the entire test tube after a few hours
- Diffusion has taken place in the liquid.
- The rate of diffusion of the particles in water is faster than the diffusion rate of particles in solid.
- The occurrence of diffusion proves that potassium permanganate(VII) consist of tiny and discrete particles.
Diffusion in Gas
 |
| (Diffusion in Gas) |
Observation
The brown colour bromine vapour spreads evenly throughout the gas jar in a few minutes
- Bromine vapour is made of tiny and discrete molecules that move randomly to fill up space.
- Bromine vapour moves randomly and diffuses in all directions in air from areas of higher concentration to areas of lower concentration.
Conclusion : The rate of diffusion is highest in gas and lowest in solid.
Brownian Motion
- Brownian motion is the physical phenomenon that tiny particles immersed in a fluid move about randomly.
- A fluid can be a liquid or a gas.
- Brownian movement, an example of diffusion, supports the kinetic theory of matter.
- Examples of Brownian movement are
- movement of smoke particles in air
- movement of pollen grains in water
Symbol of Elements
A symbol of element is the chemical symbol written in short form to represent a particular element. Some elements are represented by the first letter of its name.
Examples:
If there are two or more elements that have mane start with the same alphabet letter, a second letter is added to differentiate between these elements. The second letter used is always lowercase.
Examples:
Some elements are represented by their Latin names.
Example:
(Notes: You MUST Memorise the symbol for all these 31 elements)
| Element | Symbol |
| Fluorine | F |
| Hydrogen | H |
| Iodine | I |
| Nitrogen | N |
| Oxygen | O |
| Phosphorus | P |
| Sulphur | S |
| Carbon | C |
| Vanadium | V |
| Elements | Symbol |
| Bromine | Br |
| Calcium | Ca |
| Chlorine | Cl |
| Chromium | Cr |
| Magnesium | Mg |
| Manganese | Mn |
| Neon | Ne |
| Nickel | Ni |
| Silicon | Si |
| Helium | He |
| Argon | Ar |
| Aluminium | Al |
| Zinc | Zn |
| Platinum | Pt |
| Elements | Latin Name | Symbol |
| Copper | Cuprum |
Cu |
| Iron | Ferrum |
Fe |
| Lead | Plumbum |
Pb |
| Mercury | Hydrargyrum |
Hg |
| Potassium | Kalium |
K |
| Silver | Argentum |
Ag |
| Sodium | Natrium |
Na |
| Tin | Stannum |
Sn |
Element and Compounds
Matter can be divided into elements and compounds.


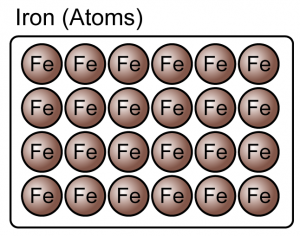
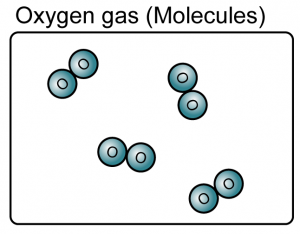
Elements
- An element is a substance that consists of only one type of atom.
- Element can be either atoms or molecules.
(Both the iron and oxygen are element because they consist of only one type of atoms)
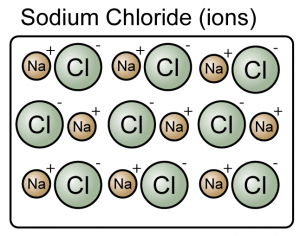
Compounds
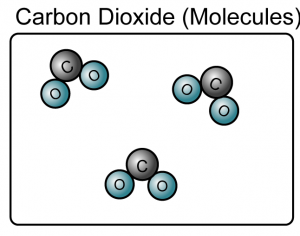
- A compound is a substance composed of molecules made up of atoms of two or more elements.
- A compound is made up of either molecules or ions.
(Both the sodium chloride and carbon dioxide are compound because they consist of more than one type of atoms)