01 Introduction to Chemistry
02 The Structure of Atoms
2.1 The Basic Concept of Matter
2 Lessons
03 Concept of Mole, Formulae and Equations
3.2 Concept of Mole
3 Lessons
3.3 Chemical Formulae
2 Lessons
04 Periodic Table of Elements
4.5 Group 17 Elements
2 Lessons
5.3 Covalent Bonds
1 Lesson
3.3.2 Formula of Ionic Compounds and Molecule
Formula of Ions
- Ionic compounds made up of positive ions and negative ions.
- To write the formula of an ionic compound, we need to know the symbol and charge of the ions in the compound.
Positive ions that you need to know in SPM form 4 chemistry:
Ion | Symbol | Ion | Symbol |
| Potassium | K+ | Calcium | Ca2+ |
| Sodium | Na+ | Magnesium | Mg2+ |
| Lithium | Li+ | Zinc | Zn2+ |
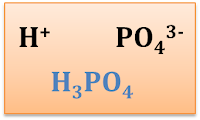
| Hydrogen | H+ | Barium | Ba2+ |
| Argentums(I) | Ag+ | Iron (II) | Fe2+ |
| Mercury(I) | Hg+ | Tin (II) | Sn2+ |
| Ammonium | NH4+ | Lead(II) | Pb2+ |
| Aluminium | Al3+ | Copper(II) | Cu2+ |
| Iron (III) | Fe3+ | Manganese(II) | Mn2+ |
Negative ions that you need to know in SPM form 4 Chemistry
Sulphate | Carbonate | Nitrate | Hydroxide |
SO42- | CO32- | NO3– | OH– |
Fluoride | Chloride | Bromide | Iodide | Oxide |
F– | Cl– | Br– | I– | O2- |
Negative ions that you need to know in SPM form 5 Chemistry
| Phosphate | PO43- |
| Ethanoat | CH3COO– |
| Manganate(VII) | MnO4– |
| Dichromate(VI) | Cr2O72- |
| Thiosulphate | S2O32- |
Chemical Formlula of Ionic Compounds
2 Requirements to form the formula of an ionic compound
- Have at least 2 types of ions that contain opposite charge.
- The amount of positive charge/charges must be equal to the amount of negative charge/charges in the compound.
Example 1 – If the Charge of the Positive Ions = Charge of Negative Ions
Write the formula of each of the following compound
- Potassium bromide
- Sodium chloride
Example 2 – If the Charge of the Positive Ions ≠ Charge of Negative Ions
Write the formula of each of the following compound
- Calcium iodide
- Sodium oxide
Example 3 – If there is more than 1 element in the ion
Write the formula of each of the compound
- Ammonium sulphate
- Zinc nitrate
Formula of Molecule
In SPM, you need to know the formulae of the following molecules
| Fluorine | F2 | Hydrogen chloride | HCl |
| Chlorine | Cl2 | Hydrogen bromide | HBr |
| Bromine | Br2 | Hydrogen iodide | HI |
| Iodine | I2 | Nitric oxide | NO |
| Carbon dioxide | CO2 | Nitrogen dioxide | NO2 |
| water | H2O | Nitrous oxide | N2O |
| Ammonia | NH3 | Tetrachloromethane | CCl4 |
| Sulphur trioxide | SO3 | Hydrogen sulphide | H2F |
| Sulphur dioxide | SO2 | Glucose | C6H12O6 |