01 Introduction to Chemistry
02 The Structure of Atoms
2.1 The Basic Concept of Matter
2 Lessons
03 Concept of Mole, Formulae and Equations
3.2 Concept of Mole
3 Lessons
3.3 Chemical Formulae
2 Lessons
04 Periodic Table of Elements
4.5 Group 17 Elements
2 Lessons
5.3 Covalent Bonds
1 Lesson
2.3.1 The Structure of Atom
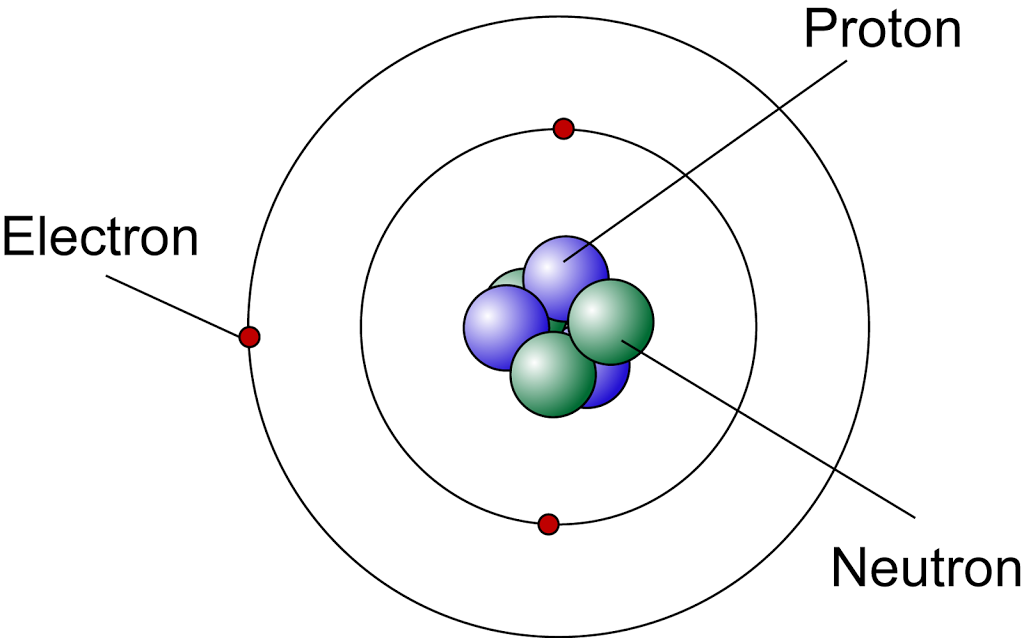
The Modern Atomic Model
According to the modern atomic model,
- The central nucleus consists of protons and neutrons. It containing almost all the mass of the atom.
- the nucleus of an atom is very small compared to the size of the atom
- the electrons are orbiting outside the nucleus in the electron shells
- the electrons are moving in electron shells at a very high speed and we cannot determine the position of the electrons at a particular time
The Subatomic Particles of an Atom
- Atoms are made up of tiny particles called subatomic particles.
- An atom contains three types of subatomic particles:
- proton,
- neutron and
- electron,
- The proton and neutron form the nucleus at the centre of an atom. They are also called the nucleon of an atom.
- The electron moves around the nucleus at a very high speed.
- The nucleus is positively charged because of the presence of protons, which are positively charged. The neutrons are neutral.
- The symbols, charge and relative masses of proton, neutron and electron are as below.
| Particle | Symbol | Relative charge | Relative mass |
| Proton | p | +1 | 1 |
| Neutron | n | 0 | 1 |
| Electron | e | -1 | 1/1840 |
The Charge of Particles
- A neutral atom contains the same number of electrons as the protons.
- The positive and negative charges of the protons and electrons respectively neutralise each other, for example, (+4) + (-4) = 0
- If the number of protons is greater than the number of electrons, the particle is positively charged.
- If the number of protons is greater than the number of electrons, the particle is positively charged.
Example:
| Number of protons | Number of electrons | Charge |
3 | 3 | 0 |
5 | 2 | +3 |
9 | 10 | -1 |
11 | 10 | +3 |
16 | 18 | -2 |
17 | 18 | -1 |
20 | 18 | +3 |
Proton Number and Nucleon Number
- Proton number = the number of protons
- Nucleon number = Number of protons + Number of neutrons
Proton Number
- The proton number (Z) represent the number of protons found in the nucleus of an atom.
- Proton number = the number of protons
- The proton number is also known as the atomic number.
- In an atom of neutral charge, the number of electrons also equals the atomic number.
- Hence, the proton number of an atom can also represent the number of electrons.
Nucleon Number
- The nucleon number (A), also called atomic mass number or mass number, is the number of protons plus the number of neutrons in an atomic nucleus. (Nucleon number = Number of protons + Number of neutrons)
- The nucleon number of an atom is about the same as the mass of the atom because the mass of an electron is very small and can be ignored.
|
Atom |
Proton Number |
Nucleon Number |
Amount of Proton |
Amount of Neutron |
| Helium |
2 |
4 |
2 |
2 |
| Oxygen |
8 |
16 |
8 |
8 |
| Sodium |
11 |
23 |
11 |
12 |
| Chlorine |
17 |
35 |
17 |
18 |
[Notes: In ion, the amount of protons IS NOT equal to the amount of electrons]