7.2.1 Factors Affecting the Rate of Reaction
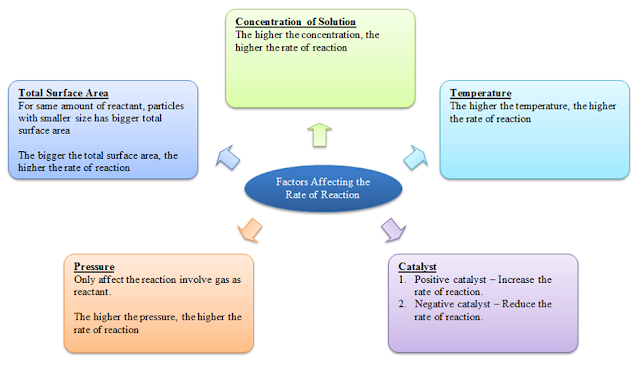
Factors Affecting the Rate of Reaction
There are 4 factors that can affect the rate of a reaction, namely
- the total surface area of the reactant (solid only)
- the concentration of the reactant (solution only)
- the temperature of the reactant
- presense of catalyst in the reactant
- the pressure of the reactant (gas only)
Total Surface Area
- For same amount of reactant, particles with smaller size has bigger total surface area
- The bigger the total surface area, the higher the rate of reaction
Concentration of Solution
The higher the concentration, the higher the rate of reaction.
Temperature
The higher the temperature, the higher the rate of reaction.
Catalyst
Positive catalyst – Increase the rate of reaction.
Negative catalyst – Reduce the rate of reaction.
Pressure
- Only affect the reaction involve gas as reactant.
- The higher the pressure, the higher the rate of reaction
Total Surface Area
The bigger the total surface area of the reactant, the higher the rate of reaction.
- For reaction involves solid reactant, when the solid reactants are broken up into smaller pieces, the total surface area of the reactant becomes bigger.
- The bigger the total surface area of the reactant, the higher the rate of reaction.
- The smaller the size of the particle, the bigger the total surface area.
- The bigger the total surface area, the higher the rate of reaction.
Experiment
The reaction between Hydrochloric acid, HCl and calcium carbonate, CaCO3.
CaCO₃ +2HCl → CaCl₂ +CO₂ + H₂O
Experiment 1
25 cm3 of 0.5 mol dm–3 hydrochloric acid + calcium carbonate chips. The carbon dioxide gas released is collected in a burrete. The volume of the gas released is recorded in every 30s. The result is plotted in a graph.
Conclusion
- The gradient of the curve for experiment 2 is greater than the curve for experiment 1. This indicate that the rate of reaction in experiment 2 is higher than experiment 1.
- We can conclude that, the smaller the particle size of the reactant, the bigger the total surface area, and the bigger the total surface area, the higher the rate of the reaction will be.
Concentration of Reactants
The higher the concentration of the solution, the higher the rate of reaction.
Experiment
- 50 cm3 of 0.2 mol dm-3 sodium thiosulphate solution + 10 cm3 of 0.5 mol dm-3 sulphuric acid.
- Time taken for the ̔X’ sign placed under the conical flask to disappear from view is recorded.
- The experiment is repeated by using sodium thiosulphate solution with concentration 0.4 mol dm-3, 0.6 mol dm-3, 0.8 mol dm-3 and 1.0 mol dm-3.
- The graph for the concentration of sodium thiosulphate (VI), Na2S2O3 against the time taken for the sulphur precipitate to formed is plotted.
- As the concentration of sodium thiosulphate solution decreases, the longer the time is needed for the marked ̔X’ to disappear.
- The graph for the concentration of sodium thiosulphate (VI), Na2S203 against 1/time taken is plotted.
- As the concentration of sodium thiosulphate increases, the value of 1/time increases. We should note that 1/time = rate of reaction.
- The higher the concentration of sodium thiosulphate solution, the higher the rate of reaction.
Temperature of the Reactant
The higher the temperature of the solution, the higher the rate of reaction.
Experiment
- 50 cm3 of 0.2 mol dm-3 sodium thiosulphate solution at 30ºC + 10 cm3 of 0.5 mol dm-3 sulphuric acid.
- Time taken for the ̔X’ sign placed under the conical flask to disappear from view is recorded.
- The experiment is repeated by using sodium thiosulphate solution with temperature 35ºC, 40ºC, 45ºC and 50ºC.
- The graph for the temperature of sodium thiosulphate (VI), Na2S2O3 against the time taken for the sulphur precipitate to formed is plotted.
- As the temperature of sodium thiosulphate solution decreases, the longer the time is needed for the marked ̔X’ to disappear.
- The graph for the temperature of sodium thiosulphate (VI), Na2S203 against 1/time taken is plotted.
- As the temperature of sodium thiosulphate increases, the value of 1/time increases. We should note that 1/time = rate of reaction.
- The higher the temperature of sodium thiosulphate solution, the higher the rate of reaction.
Presence of Catalyst
- A catalyst is a substance which can change the rate of reaction.
- There are 2 types of catalyst:
- Positive catalyst – Increase the rate of reaction.
- Negative catalyst – Reduce the rate of reaction.
Experiment 1
Set 1: Zinc + Hydrochloric Acid
Set 2: Zinc + Hydrochloric Acid + Copper Sulphate (Catalyst)
Chemical Reaction:
Result:
Conclusion
The presence of catalyst increases the rate of reaction.
Experiment 2
Set 1: Decomposition of Hydrogen Peroxide
Set 2: Decomposition of Hydrogen Peroxide + Manganese(IV) Oxide (Catalyst)
Chemical Reaction:
Result:
Conclusion:
The presence of catalyst increases the rate of reaction
Note:
In SPM, you need to remember the catalyst used in both the chemical reaction above.
Characteristic of Catalyst
- A catalyst is a substance which can change the rate of reaction.
- There are a few things you need to know about catalyst:
- Chemically, the catalyst remains unchanged during a reaction.
- Catalyst does not change the quantity of products.
- Catalyst is specific, which means different chemical reaction may have different catalyst.
- Just a small amount needed to achieve a big increase in the rate of reaction.
- More amount of catalyst used can further increase the rate of reaction.
- A catalyst in powder form can further increase the rate of reaction.
- A catalyst may undergo a physical change in a reaction.
List of Reactions and the Catalyst
| Chemical Reaction | Catalyst |
|---|---|
| Decomposition of hydrogen peroxide: 2H2O2 → 2H2O + O2 | Manganese(IV) oxide, MnO2 Lead(II) oxide, PbO Lead(IV) oxide, PbO2 |
| The reaction between Zinc and Hydrochloric Acid: Zn + 2HCl → ZnCl2 + H2 | Manganese (IV) oxide, MnO2 Copper (II) oxide, CuO Zinc Oxide, ZnO Silicon (IV) oxide, SiO2 |
| Decomposition of Potassium Chlorate (V): 2KClO3 + 2KCl → 3O2 | Copper (II) sulphate, CuSO4 Copper (II) chloride, CuCl2 Copper (II) nitrate, Cu(NO3)2 |
| Haber Process N2 + 3H2 → 2NH3 | Iron |
| Contact Process 2SO2 + O2 → 2SO3 | Vanadium(V) oxide, V2O5 |
| Ostwald Process 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(1) | Platinum |
Pressure of Gas
- For reactions involve gas, the rate of reaction is affected by the pressure of the gas.
- Pressure DOES NOT affect the rate of reaction where the reactants are in the form of solids or liquids.
- The higher the pressure of the gas, the higher the rate of reaction