05 Chemical Bonds
06 Acids, Bases and Salts
6.5 Concentration of Aqueous Solution
2 Lessons
6.9 Preparation of Salts
4 Lessons
6.11 Qualitative Analysis
3 Lessons
7.1 Determining The Rate of Reaction
2 Lessons
5.7.1 Properties of Ionic and Covalent Compounds
Ionic Compounds
Structure Ionic Compound
- In an ionic compound, the alternate positive and negative ions in an ionic solid are arranged in an orderly way as shown in the image to the right.
- The ions can form a giant ionic lattice structure with ionic bond between the ions.
- The ionic bond is the strong electrical attraction (electrostatic force) between the positive and negative ions next to each other in the lattice.
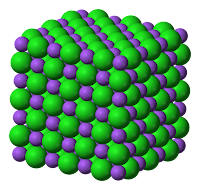 |
| (Giant Lattice Structure) |
 |
| (Strong Electrostatic Force formed between the positive and negative ions) |
Properties of Ionic Compounds
- The strong bonding force makes ionic compounds has high melting and boiling points.
- All ionic compounds are crystalline solids at room temperature.
- They are hard but brittle, when stressed the bonds are broken along planes of ions which shear away.
- Many, ionic compounds (but not all) are soluble in water.
- The solid crystals DO NOT conduct electricity because the ions are not free to move to carry an electric current.
- However, if the ionic compound is melted or dissolved in water, the liquid will now conduct electricity, as the ion particles are now free.
Covalent Compounds
Covalent compounds can be divided into 2 types:
- Simple molecular compound
- Macromolecular compound
Simple Molecules
- Most covalent compounds are made up of independent molecular units, as shown in figure above.
- The attraction force between molecules is the weak Van der Waals’ force.
Properties of Simple Covalent Molecular Substances – Small Molecules!
- The intermolecular force between the simple covalent molecules is very weak. Therefore, covalent compounds have low melting and boiling point.
- They are also poor conductors of electricity because there are no free electrons or ions in any state to carry electric charge.
- Most small molecules will dissolve in a solvent to form a solution.
Macromolecular Compounds
- The macromolecular compounds have giant, covalent molecules with extremely large molecular lattices.
- They have very high melting and boiling points.
- They don’t conduct electricity — not even when molten (except for graphite).
- They’re usually insoluble in water.
- Examples of such macromolecules are diamond, silica and graphite.
Diamond and Silica(Sand)
 |
| (3 dimensional structure macromolecular compound – Diamond) |
- A diamond crystal or a grain of sand is just one giant molecule. Such molecules, because they are so rigid and strong, have very high melting points.
- Each carbon atom forms four covalent bonds in a very rigid giant covalent structure, which makes diamond the hardest natural substance. This makes diamonds ideal as cutting tools.
- All those strong covalent bonds give diamond a very high melting point.
- It doesn’t conduct electricity because it has no free electrons.
- Diamond is an allotrope of carbon. Allotropes are different forms of the same element in the same physical state
Graphite
 |
| (3-dimensional layer structure: graphite) |
- Carbon also occurs in the form of graphite. The carbon atoms form joined hexagonal rings forming layers 1 atom thick.
- Graphite is black and opaque.
- Each carbon atom only forms three covalent bonds, creating sheets of carbon atoms which are free to slide over each other. This makes graphite slippery, so it’s useful as a lubricant.
- The layers are held together so loosely that they can be rubbed off onto paper to leave a black mark — that’s how pencils work.
- Graphite has a high melting point — the covalent bonds need lots of energy before they break.
- Only three out of each carbon’s four outer electrons are used in bonds, so there are lots of spare electrons. This means graphite conducts electricity — it’s used for electrodes.

