05 Chemical Bonds
06 Acids, Bases and Salts
6.5 Concentration of Aqueous Solution
2 Lessons
6.9 Preparation of Salts
4 Lessons
6.11 Qualitative Analysis
3 Lessons
7.1 Determining The Rate of Reaction
2 Lessons
6.9.3 Preparing Soluble Salts
Preparing Salts of Potassium, Sodium and Ammonium
- Potassium, sodium and ammonium salts are usually prepared through the reactions of acids with alkalis.
- Reaction acid with alkali will produce salt and water.Acid + Alkali → Salt + Water
- The salt is prepared by titration method of acid and alkali using an indicator.
Steps to Prepare the Salts of Potassium, Sodium and Ammonium through Titration
Step 1 – Titration to Find the End Point
- The end point is the point in a titration at which the 2 reactants have completely reacted.
- An endpoint is often marked by a color change.
Step 2 – Titrate Without Indicator
- The product obtain in step 1 is contaminate by the indicator.
- The reaction is repeated by using the same amount of reactants as in step 1, without using any indicator.
Step 3 – Crystalisation
Step 4 – Filtration and Drying
Preparing Salts of Non-"Potassium, Sodium and Ammonium"
- The salt non-potassium, sodium and ammonium is prepared by reacting acid with insoluble metal/metal oxide/metal carbonate:
- Acid + Metal Salt + Hydrogen (Displacement reaction)
- Acid + Metal oxide Salt + Water (Neutralisation Reaction)
- Acid + Metal carbonate Salt + Water + Carbon Dioxide
- Below is the steps in preparing the soluble non-potassium, sodium and ammonium salts.
Step 1 – The Reaction
Add metal/metal oxide/metal carbonate powder until excess into a fixed volume of the heated acid
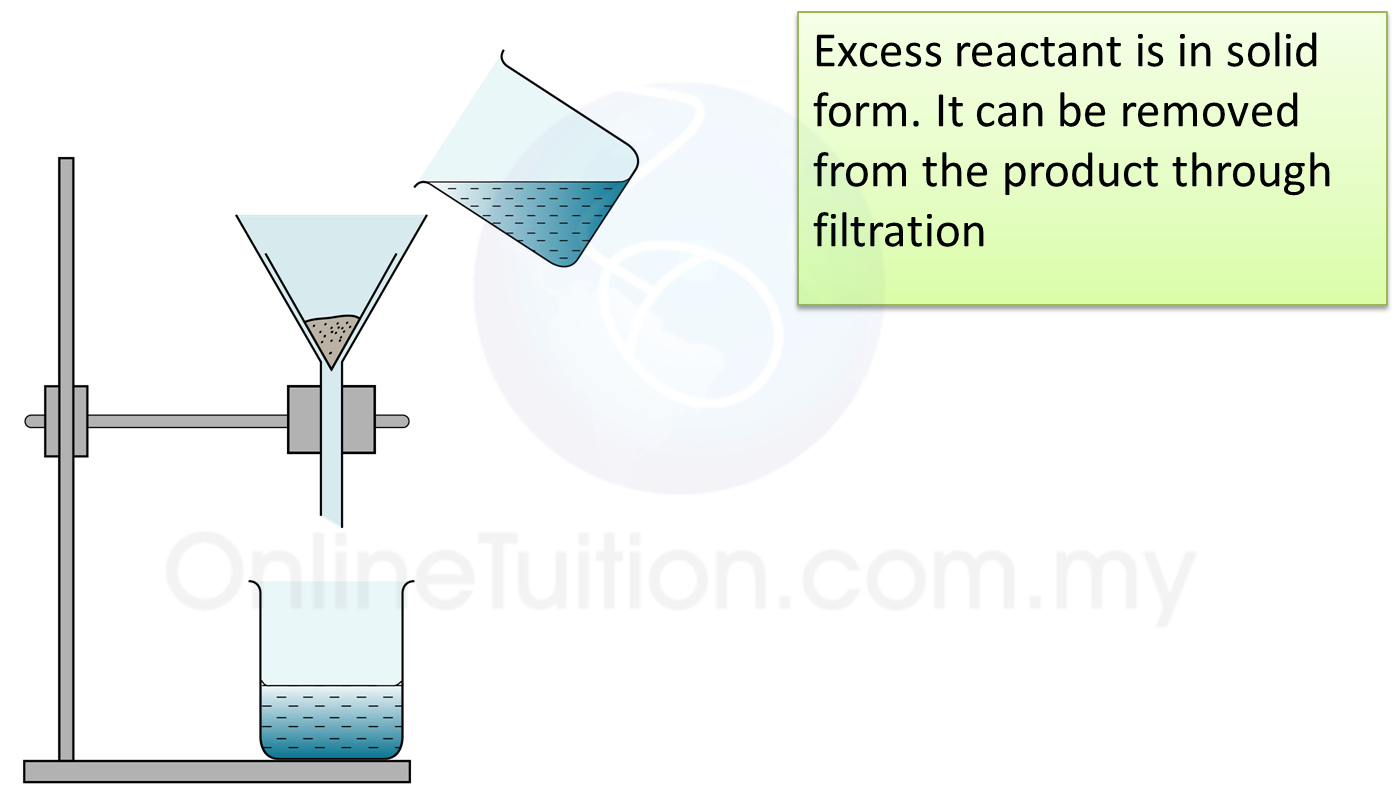
Step 2 – Filtration 1 to Remove Excess Reactant
Filter the mixture to remove excess metal/metal oxide/metal carbonate
Step 3 – Crystalisation
- Evaporate the filtrate until it becomes a saturated solution
- Dip in a glass rod, if crystals are formed, the solution is saturated.
Step 4 – Filtration 2 to Collect the Solid Salt
- Cooled at room temperature
- Filter and dry the salt crystals by pressing them between filter papers.