SBPK 2007
Exercise Summary
0 of 47 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
Information
You have already completed the exercise before. Hence you can not start it again.
Exercise is loading…
You must sign in or sign up to start the exercise.
You must first complete the following:
Results
Results
0 of 47 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- Answered
- Review
-
Question 1 of 47
1. Question
A student wants to study the relationship between the concentration of hydrochloric
acid and time of reaction by reacting the acid with magnesium ribbon.
What should be the responding variable ?CorrectIncorrect -
Question 2 of 47
2. Question
Which of the following atomic models was proposed by Ernest Rutherford?
CorrectIncorrect -
Question 3 of 47
3. Question
Avogadro number is the number of
Use the information relative atomic mass for He =4, C=12, Cl =35.5 and Ca=40CorrectIncorrect -
Question 4 of 47
4. Question
The diagram shows a graph of temperature against time for the heating of
substance X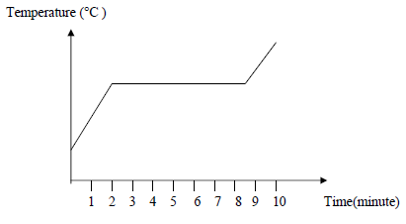
Which of the following is true about substance X at the sixth minute?CorrectIncorrect -
Question 5 of 47
5. Question
Among the following compounds, which one contains particles bonded by strong
electrostatic forces?CorrectIncorrect -
Question 6 of 47
6. Question
The table shows the proton numbers for elements X, Y and Z.
What type of oxides are formed by X, Y and Z?CorrectIncorrect -
Question 7 of 47
7. Question
Which of the following conducts electricity but does not undergo chemical
changes?CorrectIncorrect -
Question 8 of 47
8. Question
The following equation represents changes that occur during the electrolysis of
molten copper( II ) oxide.Cu2+ + me- Cu
2O2 – O2 + ne-Which set of numbers correctly represent the value of m and n?
CorrectIncorrect -
Question 9 of 47
9. Question
Which of the following substance is acidic ?
CorrectIncorrect -
Question 10 of 47
10. Question
Which of the following is not true about the properties of alkali?
CorrectIncorrect -
Question 11 of 47
11. Question
What is precipation reaction?
CorrectIncorrect -
Question 12 of 47
12. Question
What substance is embedded into glass to make a photochromic glass?
CorrectIncorrect -
Question 13 of 47
13. Question
Which of the following reactions, vanadium(V) oxide and the temperature of
450o
C are required in the Contact Process?CorrectIncorrect -
Question 14 of 47
14. Question
Aluminium powder reacts faster with hydrochloric acid than an aluminium strip
becauseCorrectIncorrect -
Question 15 of 47
15. Question
The table shows the total volume of gas collected at regular intervals in a reaction
Time (s) 0 30 60 90 120 150 180 210 Volume of gas (cm3 ) 0 2.0 3.7 5.2 6.4 7.3 8.6 8.6 What is the average rate of reaction in this experiment ?
CorrectIncorrect -
Question 16 of 47
16. Question
From the diagram above, it can be concluded that
CorrectIncorrect -
Question 17 of 47
17. Question
Which of the chemical substance can prevent the coagulation of latex?
CorrectIncorrect -
Question 18 of 47
18. Question
Which of the following is not grouped in the same homologous series ?
CorrectIncorrect -
Question 19 of 47
19. Question
The electron arrangements of atoms of elements P and Q are 2.8.4 and 2.6
respectively.
Which of the following statements about the formation of a compound of P and Q
is true ?CorrectIncorrect -
Question 20 of 47
20. Question
What is the main purpose of using superconductors?
CorrectIncorrect -
Question 21 of 47
21. Question
Which of the following is an insoluble salt?
CorrectIncorrect -
Question 22 of 47
22. Question
The diagram shows the arrangement of particles for a type of matter that
undergoes a change in physical state through process X.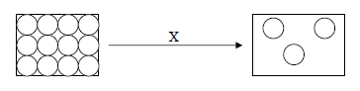
What is process X?CorrectIncorrect -
Question 23 of 47
23. Question
The table shows the melting points and boiling points of substances S, T, U, V
and W.Substance Melting point/o C
Boiling point/ o C S – 182 – 162 T – 23 77 U – 97 65 V 41 182 W 132 290 Which substance exists as a gas at room temperature?
CorrectIncorrect -
Question 24 of 47
24. Question
The table below shows the proton number of elements M and N
Element Proton number M 3 N 17 Which is true of the compound formed between elements M and N?
CorrectIncorrect -
Question 25 of 47
25. Question
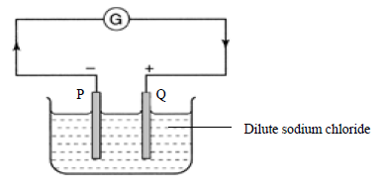
In which set of the following pair of metals would electron flow in the direction as
in the diagram?CorrectIncorrect -
Question 26 of 47
26. Question
What is the number of atoms in 1 mole of ammonia, NH3?Use the information that the Avogadro Constant 6.02 X 1023 mol-1
CorrectIncorrect -
Question 27 of 47
27. Question
The chemical formula for glucose is C6H12O6. This shows that
I the empirical formula for glucose is CH2O
II each glucose molecule is made up of 6 carbon atoms, 12 hydrogen atoms
and 6 oxygen atoms.
III 1 mol of glucose contains a total of 144 x 1023 atoms
IV One glucose molecule has a mass of 180 times higher than the mass of 1
hydrogen atom
Use the information relative atomic mass for H =1,C =12 and O =16
Avogadro Constant = 6.02 X 1023 mol-1CorrectIncorrect -
Question 28 of 47
28. Question
What is the percentage by mass of nitrogen content in urea, CO(NH2) 2 ?
Use the information that the relative atomic mass of C = 12 , N =14, H=1
and O = 16CorrectIncorrect -
Question 29 of 47
29. Question
A meteorologist would like to release a weather balloon to the amosphere to
carry out studies on the weather.Between A, B, C and D in the Periodic Table shown below which is the most
suitable element to fill up the weather balloon?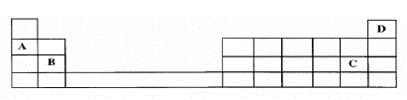 CorrectIncorrect
CorrectIncorrect -
Question 30 of 47
30. Question
The diagram shows the electron arrangement of a substance formed between two
atoms of Z.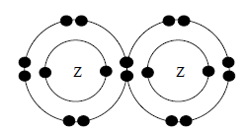
Which of the following statements is true about the substance?CorrectIncorrect -
Question 31 of 47
31. Question
When 10 cm3 of 0.50 mol dm-3 NaOH is diluted with water to 100 cm3 , the
concentration of NaOH solution isCorrectIncorrect -
Question 32 of 47
32. Question
The reaction between lead (II) nitrate and potassium iodide solution is represented
by the equation belowPb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
25.0 cm3
of 1.0 mol dm-3 potassium iodide solution is mixed with 25.0 cm3
of 1.0
mol dm-3 lead (II) nitrate solution. What is the maximum mass of lead(II) iodide
produced in this reaction?
Use the information relative atomic mass for I = 127 and Pb = 207CorrectIncorrect -
Question 33 of 47
33. Question
Which of the following is NOT a characteristic of ammonia?
CorrectIncorrect -
Question 34 of 47
34. Question
1g of magnesium was allowed to react with 50 cm3 of sulphuric acid, 1 mol dm-3.
Three experiments were conducted using three different sizes of magnesium. The
time taken to collect 20cm3 of hydrogen gas is as below.Experiment I II III Time / s 16 7 25 Which of the following experiments show the arrangement of the size of
magnesium in decreasing order?CorrectIncorrect -
Question 35 of 47
35. Question
The diagram below is a structural formula of a polymer.
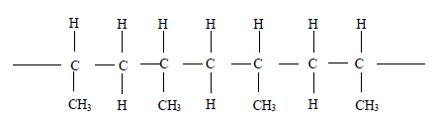
What is the structural formula of the monomer for the above polymer?CorrectIncorrect -
Question 36 of 47
36. Question
A compound with formula M2CO3 has a relative formula mass of 138.
What is the relative atomic mass of M ?
Use the information that the relative atomic mass of C = 12 and O = 16CorrectIncorrect -
Question 37 of 47
37. Question
Study the structural formula below:
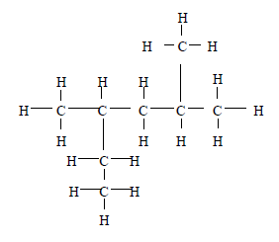
Based on the IUPAC system, what is the name of the compound having this
structural formula?CorrectIncorrect -
Question 38 of 47
38. Question
Butane has some similar characteristics with butene. The characteristics are
I Exist as gas at room temperature.
II Decolourise the purple colour of acidified potassium manganate(VII).
III Cannot dissolve in water.
IV Burn in excess air to produce a gas that turns limewater cloudy.CorrectIncorrect -
Question 39 of 47
39. Question
Two experiments were conducted to study the rate of reaction between excess
calcium carbonate and sulphuric acid as shown below.Experiment Sulphuric acid I 25cm3 H2 SO4, 1 mol dm-
3II 50cm3 H2 SO4, 0.5 mol dm –
3Which of the following graphs best describes the results of the above
experiments?CorrectIncorrect -
Question 40 of 47
40. Question
The relative atomic mass of metal Q is 7 and the relative atomic
mass of the metal W is 56Which of the following conclusions can be drawn from the above statement?
I 1 mol of W has 8 times more atoms than 1 mol of Q
II 1 atom of W is 8 times heavier than 1 atom of Q
III 1 atom of W has the same number of protons with 8 atoms of Q
IV 56 g of W has the same number of atoms as in 7 g of QCorrectIncorrect -
Question 41 of 47
41. Question
The equation shows neutralization reaction between W hydroxide solution and
nitric acid.W(OH)2 + 2HNO3 → W(NO3)2 + 2H2O
20 cm3
of W hydroxide solution 0.5 mol dm-3 neutralized 20 cm3of nitric acid.
What is the concentration of the nitric acid needed?
CorrectIncorrect -
Question 42 of 47
42. Question
An esterification reaction is given as follows:
Propanoic acid + ethanol Q + H2O
What is the molecular formula of ester Q?
CorrectIncorrect -
Question 43 of 47
43. Question
Below are the symbols of element X and Z.

If elements X and Z combine together to form an alloy Q, what is the
arrangement of atoms in alloy Q?CorrectIncorrect -
Question 44 of 47
44. Question
The equation below represents the reaction to extract aluminium from aluminium
oxide.2Al2O3 → 4Al + 3O2
What is the mass of aluminium that can be extracted from 51 g of aluminium
oxide ?
Given that relative atomic mass of Al = 27 and O = 16CorrectIncorrect -
Question 45 of 47
45. Question
Between these aqueous solutions, which will give the increasing number in pH
value?CorrectIncorrect -
Question 46 of 47
46. Question
The table shows the number of electrons, proton number and number of
neutrons for particles X and Y.Particle Number of
electronsProton number Number of
neutronsX
Y10
78
78
8Which of the following conclusions is correct for X and Y?
CorrectIncorrect -
Question 47 of 47
47. Question
The combustion of methane is as follows:
CH4 + 2O2 → CO2 + 2H2O
If 100 cm3 of methane is burnt in excess air, what is the volume of carbon
dioxide gas given off at room conditions?
Given that 1 mol of gas occupies 24 dm3 at room conditions.CorrectIncorrect