07 Rate of Reaction
7.1 Determining The Rate of Reaction
2 Lessons
08 Manufactured Substances in Industry
09 Redox Equilibrium - Practices (Coming Soon)
10 Carbon Compounds (Coming Soon)
11 Thermochemistry (Coming Soon)
12 Polymers Chemistry (Coming Soon)
13 Chemicals for Consumers and Industry (Coming Soon)
7.1.2 Analysing Rate of Reaction from Graph
Revision Progress
0% Complete
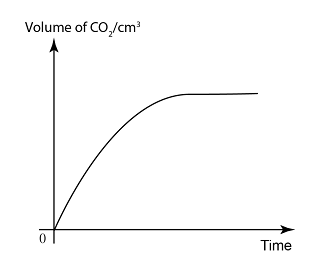
Graph of Product/Reactant Change Against Time
- In a chemical reaction,
- the reactants will decrease over time
- the product will increase over time.
- the rate of reaction will decrease over time owing to the decrease in concentration and total surface area of reactants.
- In a graph of quantity of product/reactant over time, the rate of reaction is equal to the gradient of the graph.
Example
The reaction between dilute hydrochloric acid and excess marble will produce calcium chloride and gas of carbon dioxide. Sketch the graph of
- the mass of the marble against time.
- the volume of carbon dioxide against time.
- the concentration of hydrochloric acid against time.
- the concentration of calcium chloride against time.
Answer:
a.
b.
c.
d.
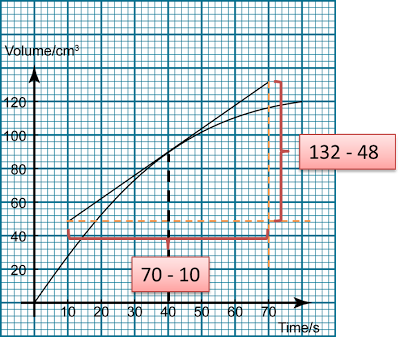
Finding the Average Rate of Reaction from a Graph
Finding Instantaneous Rate of Reaction from a Graph
- The rate of reaction changes from time to time as the reaction happens.
- The rate of reaction at a particular time is called the instantaneous rate.
- The instantaneous rate of a reaction is equal to the gradient of tangent at a particular time.
Rate of reaction = Δ(product) Δ(Time) Δ(Product) = Change of the amount of product Δ(Time) = Change of the time