03 Preparing Salts 2 &
Exercise Summary
0 of 12 Questions completed
Questions:
Information
You have already completed the exercise before. Hence you can not start it again.
Exercise is loading…
You must sign in or sign up to start the exercise.
You must first complete the following:
Results
Results
0 of 12 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 12
1. Question
1 point(s)CHEM0803010
A pure sample of the insoluble salt barium carbonate can be made using the method given.
Which instruction is missing from step 5?CorrectIncorrect -
Question 2 of 12
2. Question
1 point(s)CHEM0803020
Four steps in the preparation of a salt from an acid and a solid metal oxide are listed.- Add excess solid
- Evaporate half the solution and leave to cool
- Filter to remove unwanted solid
- Heat the acid.
In which order should the stages be carried out?
CorrectIncorrect -
Question 3 of 12
3. Question
1 point(s)CHEM0803030
Zinc chloride is made by reacting an excess of zinc oxide with dilute hydrochloric acid. The excess zinc oxide is then removed from the solution. Which process is used to obtain solid zinc chloride from the solution?CorrectIncorrect -
Question 4 of 12
4. Question
1 point(s)CHEM0803040
Which substance reacts with dilute sulfuric acid to produce a salt that can be separated from the resulting mixture by filtration?CorrectIncorrect -
Question 5 of 12
5. Question
1 point(s)CHEM0803050
Four steps in the preparation of a salt from an acid and a solid base are listed.- Neutralisation
- Filtration
- Crystallisation
- Evaporation
In which order are the steps carried out?
CorrectIncorrect -
Question 6 of 12
6. Question
1 point(s)CHEM0803060
Which two compounds give a white precipitate when their aqueous solutions are mixed?CorrectIncorrect -
Question 7 of 12
7. Question
1 point(s)CHEM0803070
The table below shows the solubility of some silver compounds.Compound Solubility in Water Silver Oxide Insoluble Silver Carbonate Insoluble Silver Chloride Insoluble Silver Nitrate Soluble Which equation shows a reaction which cannot be used to prepare a silver salt?
CorrectIncorrect -
Question 8 of 12
8. Question
1 point(s)CHEM0803080
How many different salts could be made from a supply of dilute sulfuric acid, dilute hydrochloric acid, zinc, aluminium oxide and copper(II) carbonate?CorrectIncorrect -
Question 9 of 12
9. Question
1 point(s)CHEM0803090
A student found that when he mixed solution P and solution Q in a test tube, some white precipitate formed immediately. Which of the following are possibly solution P and solution Q?CorrectIncorrect -
Question 10 of 12
10. Question
1 point(s)CHEM0803100
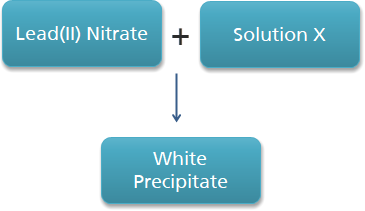
The diagram above shows the reaction of lead(II) nitrate with solution X. Solution X might be
CorrectIncorrect -
Question 11 of 12
11. Question
1 point(s)CHEM0803110
A solution contains barium ions and silver ions. What could the anion be?CorrectIncorrect -
Question 12 of 12
12. Question
1 point(s)CHEM0803120
Which of the following are double decomposition reaction to prepare insoluble salts?CorrectIncorrect