Basic Structure
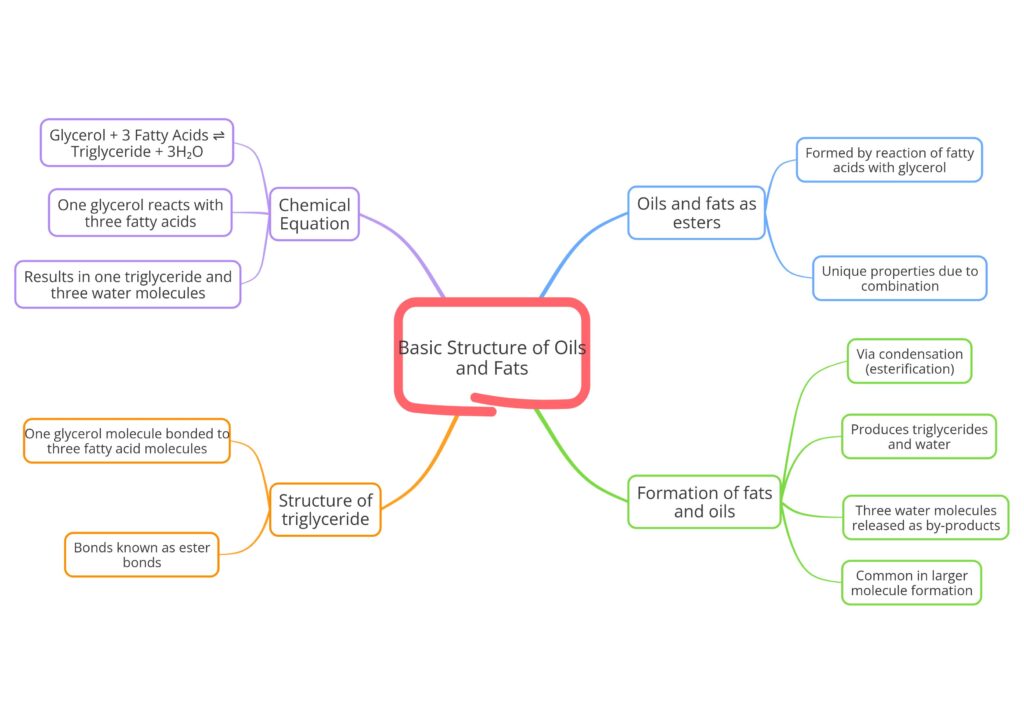
Esters Formation: Oils and fats belong to a group of compounds called esters. These esters are created through a special chemical reaction where molecules called fatty acids combine with another molecule called glycerol. This combination creates a new substance with unique properties.
Condensation Reaction: The process where fatty acids and glycerol join together is called a condensation reaction, also known as esterification. During this reaction, three water molecules are released as by-products. This type of reaction is common when making larger molecules from smaller ones.
Triglyceride Composition: The final product of the reaction between glycerol and fatty acids is called a triglyceride. A triglyceride is made up of one glycerol molecule that is chemically bonded to three fatty acid molecules. These bonds are called ester bonds.
Chemical Equation: This reaction can be shown as a chemical equation: Glycerol + 3 Fatty Acids ⇌ Triglyceride + 3H₂O. This means that one glycerol molecule reacts with three fatty acids to form one triglyceride and three molecules of water.
Fatty Acids
Carboxylic Acids: Fatty acids are a special kind of acid called carboxylic acids. These acids have long chains made of carbon and hydrogen atoms. Most fatty acids have between 12 to 18 carbon atoms, making them long molecules.
Examples of Fatty Acids: Some well-known examples of fatty acids include stearic acid, which is found in animal fats, oleic acid, which is found in olive oil, and palmitic acid, which is common in palm oil. Each of these fatty acids has different properties depending on their structure.
Saturation Types: Fatty acids can be classified into two main types: saturated and unsaturated. Saturated fatty acids only have single bonds between carbon atoms in their chains. Unsaturated fatty acids have one or more double bonds between carbon atoms, which affects their shape and how they behave at room temperature.
Glycerol
Structure of Glycerol: Glycerol is a type of alcohol. Its scientific name is propan-1,2,3-triol, which means it has three carbon atoms, each attached to a hydroxyl group (-OH). These hydroxyl groups are the parts that react with fatty acids to form ester bonds, making glycerol an important part of the triglyceride structure.
Triglycerides
Main Form of Storage: In living things like humans, animals, and plants, triglycerides are the most common form of stored fat. They are found in fat tissue and plant oils and act as a way to keep energy for future use.
Ester Linkage: Triglycerides are formed through three ester linkages. Each hydroxyl group on the glycerol reacts with the acid group on a fatty acid. This forms three ester bonds, creating a stable triglyceride molecule.
General Formula: The structure of a triglyceride can be written as (CH₂-O-CO-R₁) – (CH-O-CO-R₂) – (CH₂-O-CO-R₃). In this formula, CH₂ and CH refer to parts of the glycerol, and R₁, R₂, and R₃ represent the hydrocarbon chains from the three fatty acids, which can be different or the same.
Types of Fats and Oils
Physical State Difference: Fats and oils can be told apart by how they look and feel at room temperature. Fats are usually solid or semi-solid, like butter or lard. Oils are liquid, like cooking oil or olive oil.
Source of Oils: Oils are mainly obtained from plants. For example, coconut oil comes from coconuts, olive oil from olives, and corn oil from corn. These oils are liquid because they often contain unsaturated fatty acids.
Source of Fats: Fats mostly come from animals. Some common examples include butter, which is made from cream, cheese, which is made from milk, and animal fat from meat. These fats usually contain more saturated fatty acids, which makes them solid.
Saturated Fats: These fats have fatty acids with no double bonds between the carbon atoms. Because of this structure, the molecules can pack tightly together, making the fat solid at room temperature.
Unsaturated Fats: These fats have one or more double bonds in their fatty acid chains. The double bonds create bends in the chains, preventing the molecules from packing closely, which keeps the fat liquid.
Monounsaturated Fats: This type of fat contains only one double bond in its fatty acid chain. Olive oil is an example of a fat that is rich in monounsaturated fatty acids.
Polyunsaturated Fats: These fats have two or more double bonds in their carbon chains. They are found in foods like sunflower oil, fish oil, and walnuts. They are usually the healthiest type of fat.
Key Reactions
Hydrolysis Reaction: This is a reaction where triglycerides are broken down into glycerol and fatty acids. It happens when water is added to the triglyceride, and it can be sped up using an acid or a base (alkali). This reaction is important for digestion.
Hydrogenation Process: Hydrogenation is a chemical reaction where hydrogen gas is added to unsaturated fats. This changes the double bonds into single bonds, turning the fat from a liquid into a solid. The reaction needs a nickel catalyst and happens at high temperature and pressure.
Margarine Production: In the food industry, hydrogenation is used to turn vegetable oils, which are liquid, into margarine, which is semi-solid. This process helps make margarine spreadable and increases its shelf life.
Uses and Importance in the Body
Energy Source: Fats and oils are one of the body’s main sources of energy. They provide more than twice the energy per gram compared to carbohydrates and proteins
Energy Storage: When the body has extra energy from food, it stores it as fat. This stored fat can be used later when the body needs more energy.
Insulation and Protection: Fat in our body helps keep us warm by acting as insulation. It also cushions and protects our internal organs, like the heart and kidneys, from physical damage.
Vitamin Transport: Some vitamins can only dissolve in fat, not in water. These are called fat-soluble vitamins and include vitamins A, D, E, and K. Fats help carry these vitamins in the bloodstream so the body can use them.
Health Concerns
LDL Cholesterol Risk: Eating too much saturated fat can raise the level of a type of cholesterol called LDL, or “bad cholesterol,” in the blood. High LDL levels can block blood vessels and increase the risk of heart disease.
Trans Fats Effect: Trans fats are a type of fat that is made during the hydrogenation process. They are harmful because they raise LDL cholesterol and lower HDL, or “good cholesterol,” increasing the risk of heart attacks and strokes.
Obesity Link: When a person eats more fat than their body needs, the extra fat is stored, leading to weight gain. Too much body fat can lead to obesity, which increases the risk of other health problems like diabetes and high blood pressure.